CO2 laser beam melting of aluminum oxide (Al2O3).
This experiment illustrates laser beam melting of levitated aluminum oxide. Time interval = 5.6 seconds. Solid alumina remains at the bottom of the sample for 3.4 seconds. Stirring by the levitation gas flow increases when melting is complete.
This experiment illustrates laser beam melting of levitated aluminum oxide. Time interval = 5.6 seconds. Solid alumina remains at the bottom of the sample for 3.4 seconds. Stirring by the levitation gas flow increases when melting is complete.
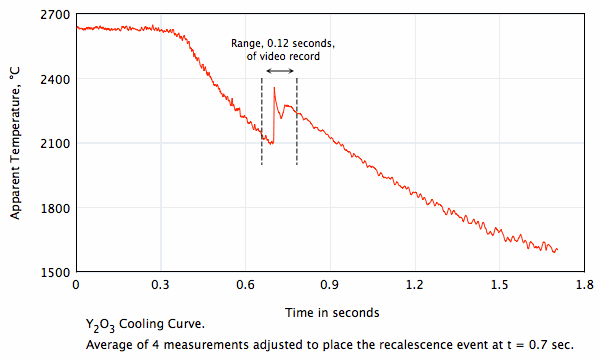
Undercooling, solidification, and crystal phase transformation of Y2O3.
Frame rate = 2,600/second.
- A crystal phase transformation occurs at T > 2275 °C
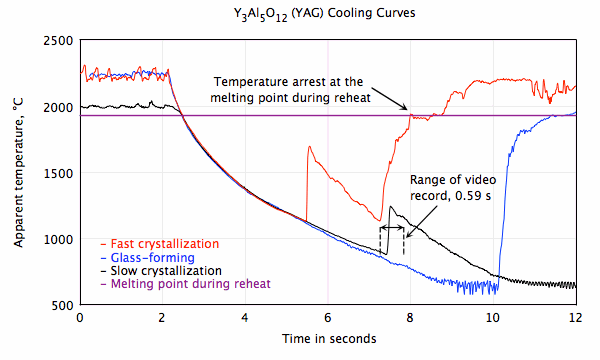
Undercooled liquid Y3Al5O12 (YAG) slow
crystallization. Depending on sample size and cooling rate, liquid YAG may:
- Crystallize spontaneously with recalescence in less than 3 ms at an apparent temperature, TA ≈ 1000 °C.
- Fail to crystallize and form a glass product.
- An unusual result was obtained in this experiment. The liquid cooled to TA ≈ 880 °C, then crystallized slowly in a period of approximately 0.5 seconds.
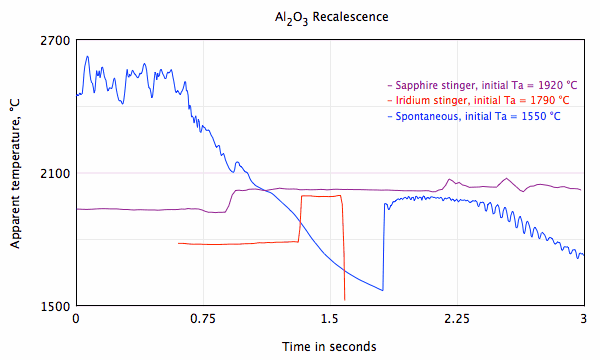
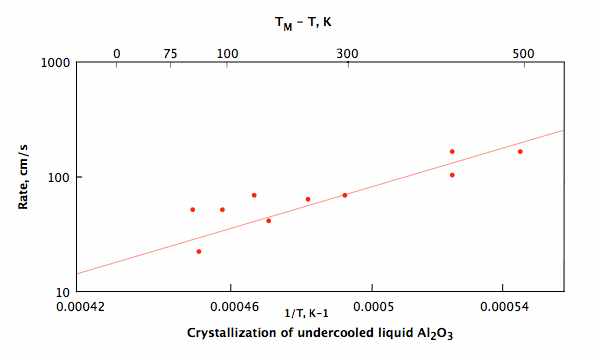
Crystallization of undercooled aluminum oxide

Spontaneous nucleation of solid and rapid crystallization at deep undercooling.
Initial apparent temperature = 1550 °C
Initial apparent temperature = 1550 °C
Crystallization at moderate undercooling initiated with an iridium "stinger"
Initial apparent temperature = 1790 °C
Initial apparent temperature = 1790 °C

Crystallization at slight undercooling initiated with a sapphire "stinger"
Initial apparent temperature = 1925 °C
Initial apparent temperature = 1925 °C